PRODUCT CENTERMachines supportPRODUCT CENTER/ Machines supportHot Melt Extruder
Purpose: This device is an exclusive solid dispersion one-step granulator developed by our company for pharmaceutical formulations. It can achieve one-step granulation of various types of solid dispersions such as taste masking, solubilization assistance, and targeted release, and can also directly prepare slow-release granules. Principle: The raw materials are fed into the feeding hopper through a quantitative feeding device and then evenly conveyed by a twin-screw. The screw is heated in sections, gradually increased in temperature, and the material is melted step by step. In the middle section, it achieves fusion, and then gradually decreases in temperature. Finally, it is extruded by the screw through the orifice plate to form a dense and hard strip. The target particle size product is obtained by conveying and shearing the strip material through a conveying and shearing device. Characteristic: 1. Feeding system: vibration feeder, twin-screw feeder, to meet the feeding needs of customers for materials of different properties.2. Heating method: Design to perform segmented electric heating on both upper and lower cylinders simultaneously, ensuring uniform heating. 3. Multi stage precise temperature control system (within the set value range of ± 0.5 ℃): adopting a precise temperature control feedback system, the temperature of each section is stable without interference, ensuring the stability and reproducibility of the hot melt process. 4. Current monitoring feedback system: online monitoring of extrusion current value, determination of extrusion torque, feedback protection of twin-screw, and increase screw service life. 5. Dual cooling system: integrating water cooling and air cooling, better ensuring the stability heating temperature. 6. Combination twin-screw: The twin-screw adopts a multi-stage splicing form, integrating mixing, pressurization, melting, extrusion, and cooling to ensure complete fusion of the main drug and auxiliary materials. 7. Orifice plate selection: Different shapes and apertures of extrusion orifice plates can be selected to adapt to the extrusion of materials with different properties. 8. Multi stage exhaust: Design multi-stage exhaust holes to effectively discharge air, moisture, etc. from the molten material, reduce extrusion pressure, and improve product quality. 9. Conveyor shearing device: Different structures of shearing devices can be equipped according to the characteristics of different materials to obtain uniform particles. 10. Compliant with GMP standards, easy to disassemble and clean, with three-level password permissions. Process parameters are automatically controlled by PLC, and all data is automatically stored online and can be exported via USB flash drive. 11. Function extension: Continuous wet granulation. Specifications-models-main technical parameters:
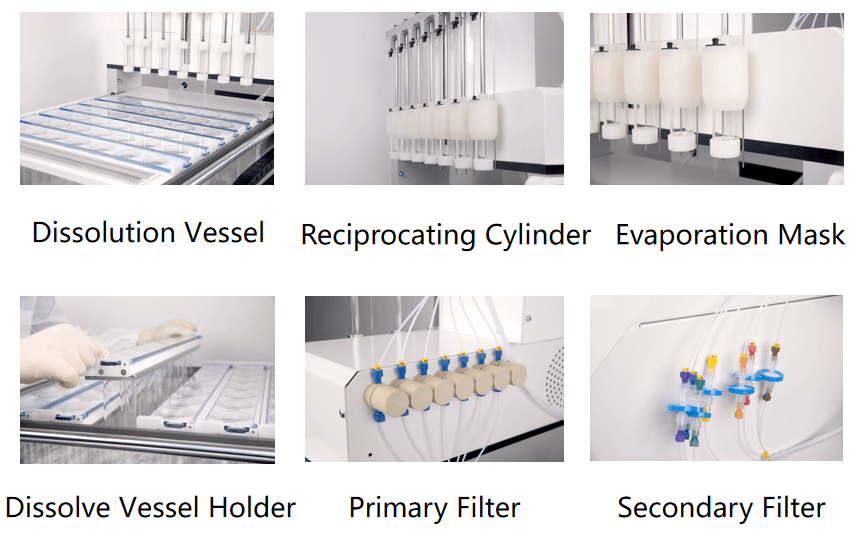
DS-BIOAT Reciprocating Cylinder Product Introduction:The DS-BIOAT automatic reciprocating cylinder dissolution system consists of a reciprocating cylinder dissolution device and an automatic sampling workstation. Meet the relevant requirements of the US Pharmacopoeia, Chinese Pharmacopoeia and European Pharmacopoeia. The device adopts a transparent hollow cylinder equipped with screens at both ends. The sample to be tested is placed in the cylinder and the screens at both ends are tightened before being placed in a dissolution cup containing dissolution medium and gently reciprocating up and down. The dissolution medium flows through a reciprocating cylinder through a sieve, providing shear force at the liquid-solid interface during the dissolution process of the drug. Suitable for the dissolution study of new drug formulations such as sustained-release, targeted release, and enteric coated delayed release formulations. Main Features: 1. 300ml glass dissolution cup, 7-position, 6-row, in accordance with pharmacopoeia requirements. 2. The connecting rod type reciprocating structure ensures that the reciprocating stroke is fixed and unchanged. 3. Better leakage conditions have better dissolution effects for low solubility drugs. 4. The standard design meets the requirement of fully automatic switching between multiple media, better simulating changes in human pH environment. 5. Less media required, easy to change pH and good correlation with bioavailability data. 6. Online filtration function: two-stage filtration, columnar filtration, and 0.45um membrane circulation filtration. The 0.45um membrane is installed in the circulation pipeline to fully solve the problem of sample adsorption. During the experiment, no air is allowed to pass through the membrane, ensuring stable system pressure. 7. software system or enterprise servers.
|